
Why Is Medical Product Training Important?
Medical product training is important for several reasons.
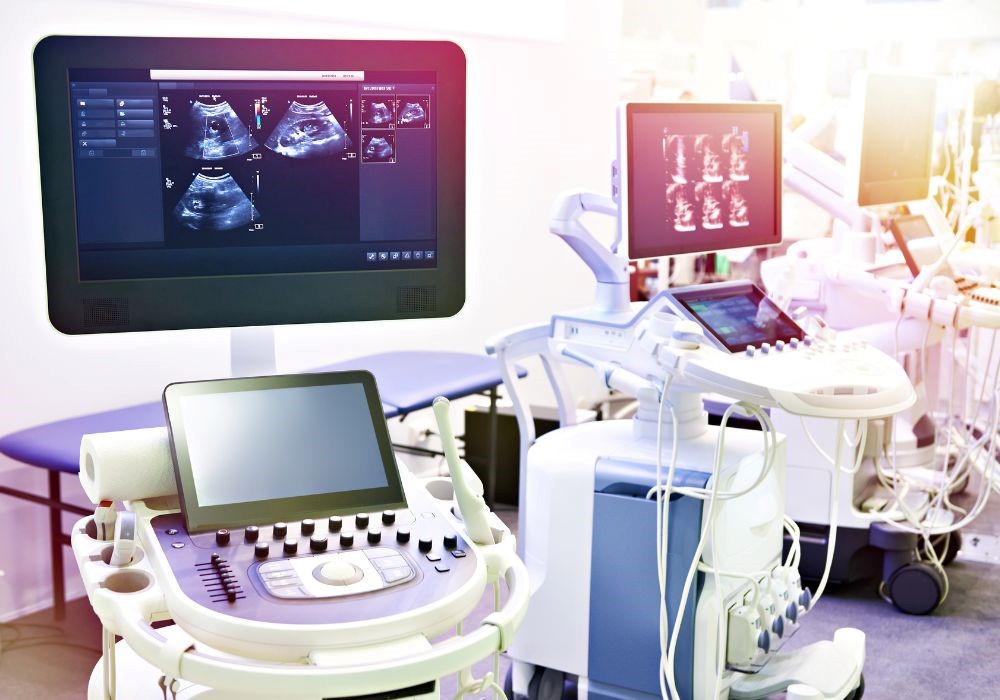
Мedical staff need to be able to properly use and maintain the equipment. Medical product training helps ensure that patients receive the best possible care, medical product training can help prevent errors and improve patient safety.
Medical product training helps keep costs down by reducing the need for repairs and replacements.
Medical Devices And Their Use
Medical devices are designed to help diagnose, treat, or prevent medical conditions.
They range from simple devices, such as tongue depressors and bandages, to complex devices, such as pacemakers and heart-lung machines.
Some medical devices are used for diagnostic purposes – X-ray machines and computed tomography (CT) scanners help doctors to see inside the body.
Other diagnostic devices include ultrasound machines and MRI machines.
Medical devices can also be used to treat medical conditions – stents are often used to prop open arteries that have been blocked by plaque.
Pacemakers are used to treat heart arrhythmias. Dialysis machines are used to treat kidney failure.
Some medical devices are used to prevent medical conditions – condoms can help to prevent the spread of sexually transmitted diseases. Immunization shots help to prevent certain infections. Fluoride treatments help to prevent tooth decay.
Medical devices must be safe and effective for their intended use. The U.S. Food and Drug Administration (FDA) is responsible for regulating medical devices.
Manufacturers must submit their devices to the FDA for approval before they can be marketed to the public.
The FDA has four main types of medical device regulations: premarket notification (510(k)), premarket approval (PMA), investigational device exemption (IDE), and humanitarian device exemption (HDE).
Premarket notification (510(k)) is the most common type of regulation for medical devices. In order to receive 510(k) clearance, manufacturers must demonstrate that their device is “substantially equivalent” to a device that is already on the market.
Premarket approval (PMA) is the second most common type of regulation for medical devices. In order to receive PMA approval, manufacturers must demonstrate that their device is safe and effective for its intended use.
Investigational device exemption (IDE) is necessary for clinical studies of investigational devices.
An IDE allows manufacturers to conduct clinical studies of their devices in order to collect data on safety and effectiveness.
Humanitarian device exemption (HDE) is necessary for devices that are intended to treat or diagnose a rare disease or condition.
In order to receive HDE approval, manufacturers must demonstrate that their device is probable to benefit patients with the disease or condition.
The FDA also has postmarket surveillance programs in place to monitor the safety and effectiveness of medical devices that are already on the market. These programs help to ensure that devices remain safe and effective for patients.
Medical Product Training
- medical product training is important because it ensures that healthcare professionals are able to use the products safely and effectively
- it can help to improve patient outcomes
- It can also help to reduce costs by ensuring that products are used in the most cost effective way possible.
- In some cases, medical product training may be required in order to obtain insurance coverage for the use of a particular device
- Мedical product training may be required by accreditation bodies in order for a healthcare facility to maintain its accreditation
- In some cases, medical product training may be mandated by state or federal regulations.
- Medical product training may also be recommended or required by the manufacturer of a particular device.
There are many different types of medical products on the market, and each one requires specific training in order to be used safely and effectively.
New technologies such as robotic surgery require specialized training for surgeons in order to ensure that they are able to use the equipment safely and effectively.
Similarly, new drugs and medical devices also require specific training for healthcare professionals in order to ensure that they are able to use them safely and effectively.
Medical product training is therefore essential for all healthcare professionals who use medical products, in order to ensure that they are able to use them safely and effectively.
In addition, medical product training can also help to improve patient outcomes by ensuring that products are used in the most effective way possible. Medical product training can also help to reduce costs by ensuring that products are used in the most cost effective way possible.
There are many different ways in which medical product training can be delivered, such as through online courses, face-to-face courses, or hands-on training.
However, it is important to ensure that the training is delivered by experienced and qualified instructors in order to ensure that it is effective.
The training should be tailored to the specific needs of the healthcare professionals who will be using the products.
Medical product training is therefore an essential part of ensuring that healthcare professionals are able to use medical products safely and effectively.
If you are a healthcare professional who uses medical products, it is important that you receive the appropriate training in order to ensure that you are able to use them safely and effectively.
Takeaway
Medical product training is essential for proper patient care. It is important to find a program that covers the products you use regularly and to make sure the instructors are qualified. With the right training, you can be confident you are using your products safely and effectively.


